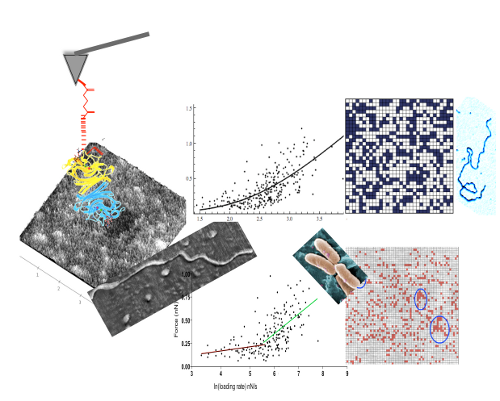
Mucins are highly glycosylated molecules participating in the formation of the glycocalyx and of gels that line, for example, the eye, or the respiratory and gastrointestinal tracts. Commensal bacteria play an important role in mucin gel turnover, binding to and degrading mucins, while pathogenic organisms that bind to surface epitopes cause infection and disease. Microorganisms have an exquisite selectivity for binding partners: a sialic acid might be adhesive or not depending on its linkage to the preceding sugar in the chain. Using atomic force microscopy (AFM) we characterised the distribution of sialic acids and mucin peptide core epitopes to understand the packaging of mucins in ocular surface gels and assess the balance of pro- and anti-adhesive epitopes at the gel surface. Human ocular mucins were purified using isopycnic centrifugation and gel filtration. A small sample of preocular gel was removed from the lower lid, kept hydrated at all times and imaged in liquid. AFM tips were functionalised, through flexible linkers, with lectins (to probe sialic acids), and antibodies (to assess the presence and configuration of mucins). The frequency and distribution of each of the epitopes was analysed from topographic and adhesion maps obtained using a Dimension AFM with a Nanoscope IV controller (Veeco, USA) in Force Volume mode. For each bioprobe molecular bond characteristics (rate of dissociation, and potential width) were compared between purified mucins and mucus gels to evaluate any effects of the molecular environment. Clustering of epitopes was established by comparison with randomly-generated distributions. Irrespective of mucin molecules being deposited on mica or part of a gel, lectins unbinding from sialic acids best fit a double energy barrier model, while unbinding forces between antibodies and the mucin peptide cores show an exponential relationship with the loading rate not dependent on discrete transition states. The ratio of α2,3– to α2,6–linked sialic acids is reversed at the surface of the preocular gel compared with purified mucins. Far fewer clusters of α2,3-linked sialic acids are seen on the gel surface than on mucin molecules, while the opposite is true for α2,6 – linked sialic acids, which are more numerous and have more neighbouring interactions on the gel surface than in purified mucins. Clusters of binding sites, significantly more often than expected at random, are numerous on surface of gels, but represent only a fraction of the contour length of a mucin, indicating that mucins are not fully extended, or that core epitopes are shielded by other entities in the gel. When pulled through, or out of, the network, gel forming mucins extend further than the shed cell surface associated mucin MUC16. Through interactions with richly expressed sugars (α2,3-linked sialic acids), mucins move further through or with the gel than from the mica surface. Mucin molecules are not fully exposed at the surface of the preocular gel, a configuration that could protect from proteolytic cleavage by external organisms. Furthermore, for the ocular surface, the surface is largely anti-adhesive for Pseudomonas aeruginosa. For this pathogen, the few receptor clusters on mucins are likely to promote elimination from the ocular surface.